Hello everyone........
Welcome to FWQRC
Today’s topic is related to documentation requirements at each stage of process validation life cycle
- Documentation is important so that knowledge gained about a product and process is accessible and comprehensible to others involved in each stage of the process validation life cycle
- They are essential to enabling organizational units responsible and accountable for the process to make informed, science based decisions that ultimately support the release of a product to commerce
- The degree and type of documentation required cGMP vary during the validation life cycle
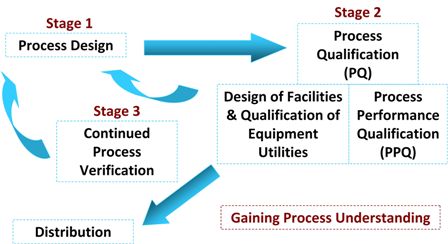
Stage-1:
- cGMP documentation for commercial manufacturing (i.e., initial commercial master batch production and control record (21 CFR part 211.186) and supporting procedures) are key outputs of stage-1, process design
Stage-2 and Stage-3:
- Documentation requirements are greatest during stage-2 process qualification and stage-3 continued process verification
- Studies during these stages must confirm to the CGMPs and must be approved by the quality unit in accordance with the regulations (21 CFR part 211.22 and 211.100)
- Viral and impurity clearance studies, even when performed at small scale, also require quality unit oversight
- Process flow diagrams should describe in each unit operations, its placement in the overall process, monitoring and control points and the component, as well as other processing material inputs (e.g. processing aids) and expected outputs (e.g. in-processing material and finished product)
- To generate and preserve process flow diagrams of the various scales as the process designs progress to facilitate comparison and decision making about their comparability
Thank you for reading our blogs. See you soon with another content