Hello Everyone...
Greetings of the day
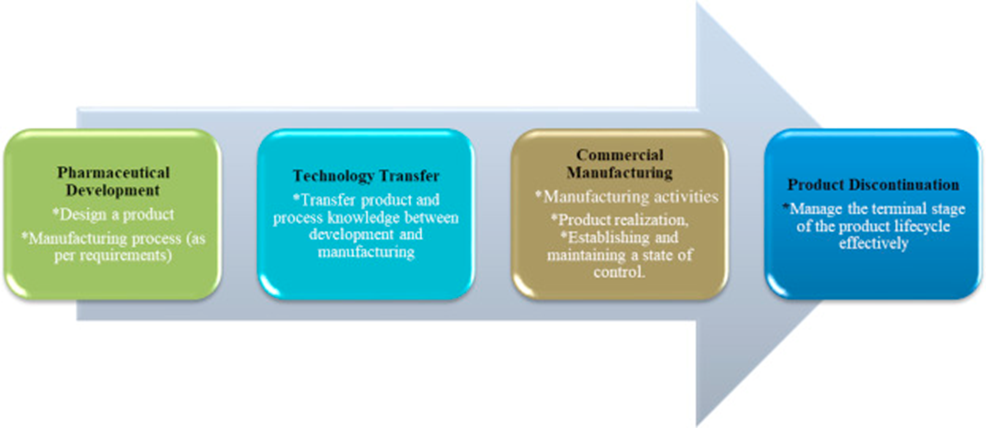
Today’s topic is “Application of Change management system throughout the product life cycle”
Pharmaceutical Development:
Change is an inherent part of the development process and should be documented; the formality of the change management process should be consistent with the stage of pharmaceutical development
Technology transfer:
The change management system should provide management and documentation of adjustments made to the process during technology transfer activities
Commercial manufacturing:
A formal change management system should be in place for commercial manufacturing. Oversight by the quality unit should provide assurance of appropriate science and risk based assessments
Product discontinuation:
Any changes after product discontinuation should go through an appropriate change management system